 |
OraQuick HCV
ORASURE TECHNOLOGIES, INC.
The OraQuick® HCV Rapid Antibody Test is the FIRST and ONLY FDA-approved, point of care test for the detection of HCV antibodies. Our simple, CLIA-waived platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes. The test can be completed in 3 simple steps from 5µl of fingerstick or venous whole blood with less than 2 minutes of hands on time.
|
|
The OraQuick® HCV Rapid Antibody Test is the FIRST and ONLY FDA-approved, point of care test for the detection of HCV antibodies. Our simple, CLIA-waived platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes. The test can be completed in 3 simple steps from 5µl of fingerstick or venous whole blood with less than 2 minutes of hands on time. It is ideal for testing programs in both clinical and non-clinical settings, as well as outreach events. Examples include urgent care, labor and delivery, laboratories and physician offices  OraQuick HCV Rapid Antibody Test The FIRST and ONLY FDA- approved, CLIA-waived test for HCV antibodies Accurate
Simple
Fast
ORASURE TECHNOLOGIES, INC.
220 East First Street | | Bethlehem | PA |
Based in Bethlehem, Pennsylvania, OraSure Technologies
develops, manufactures and markets point-of-care, oral fluid specimen collection
devices that leverage proprietary oral fluid technologies, diagnostic products,
including immunoassays and other in vitro diagnostic tests, and other medical devices.

|
|
 |
OraSure Ora Quick Advance HIV-1/2
ORASURE TECHNOLOGIES, INC.
The OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test is an FDA-approved point of care screening test for use with oral fluid, fingerstick or venous whole blood and plasma. The test delivers lab accurate results within 20 minutes with less than 2 minutes of hands on time. CDC guidelines call for routine testing in all healthcare settings to identify all HIV positive people and connect them to care.
|
|
The OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test is an FDA-approved point of care screening test for use with oral fluid, fingerstick or venous whole blood and plasma. The test delivers lab accurate results within 20 minutes with less than 2 minutes of hands on time. CDC guidelines call for routine testing in all healthcare settings to identify all HIV positive people and connect them to care. This simple test is ideal for testing programs in both clinical and non-clinical settings, as well as outreach events. Examples include urgent care, labor and delivery, laboratories and physician offices.  OraQuick ADVANCE HIV-1/2 Rapid Antibody Test The early HIV antibody detection test for recent infection Sensitivity
Versatility
Reliability
ORASURE TECHNOLOGIES, INC.
220 East First Street | | Bethlehem | PA |
Based in Bethlehem, Pennsylvania, OraSure Technologies
develops, manufactures and markets point-of-care, oral fluid specimen collection
devices that leverage proprietary oral fluid technologies, diagnostic products,
including immunoassays and other in vitro diagnostic tests, and other medical devices.

|
|
 |
cobas® Influenza A/B assay
ROCHE DIAGNOSTICS
The cobas Influenza A/B assay is the first CLIA-waived, real-time PCR test to detect Influenza A and B in ~20 minutes
|
|
cobas® Influenza A/B assay The cobas® Influenza A/B assay is the first CLIA-waived, real-time PCR test to detect Influenza A and B in ~20 minutes. Available for use in non-traditional testing sites, including ERs, physician offices, pharmacy clinics and other urgent care settings. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
|
 |
cobas® Strep A assay
ROCHE DIAGNOSTICS
The cobas Strep A assay is a CLIA-waived, real-time PCR test for the detection of Streptococcus group A in throat swab specimens from patients with signs and symptoms of pharyngitis.
|
|
cobas® Strep A assay The cobas® Strep A assay is a CLIA-waived, real-time PCR test for the detection of Streptococcus group A in throat swab specimens from patients with signs and symptoms of pharyngitis. With excellent sensitivity and specificity, this assay provides the reassurance needed when deciding whether or not to treat with antibiotics. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
|
 |
cobas® Influenza A/B & RSV assay
ROCHE DIAGNOSTICS
The cobas Influenza A/B & RSV assay is the first CLIA-waived, real-time PCR test that differentiates Influenza A, Influenza B and RSV in 20 minutes
|
|
cobas® Influenza A/B & RSV assay The cobas Influenza A/B & RSV assay is the first CLIA-waived, real-time PCR test that differentiates Influenza A, Influenza B and RSV in 20 minutes. It's available for use in hospitals, physician offices and urgent care settings. Benefits:
ROCHE DIAGNOSTICS
9115 Hague Road | Bldg. H | Indianapolis | IN |
Personalised Helathcare (PHC) is based on the observation that patients with the same diagnosis react to the same treatment in different ways

|
|
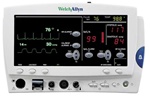 |
Welch Allyn Atlas Monitor w/ECG, Nellcor SpO2 & NIBP (621 Series)
MEDICAL DEVICE DEPOT
A cost-effective monitor with all the parameters for conscious sedation procedures.
|
|
Simple, straightforward menu operation
MEDICAL DEVICE DEPOT
3230 Bethany Lane | Suite 8 | Ellicott City | MD |
Medical Device Depot is committed to making your on- and off-line shopping experience with us worry-free.

|
|
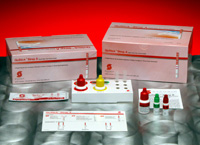 |
STREP A Rapid Strip
STANBIO LABORATORY
Stanbio Laboratory offers the QuStick™ Strep A Rapid Strip test, a quickstep test designed for the qualitative detection of strep A antigen directly from throat swab specimens. Stanbio’s accurate device provides test results in a matter of minutes, which allows physicians to make confident treatment decisions while the patient is in the office. It utilizes antibodies specific for whole cell Lancefield group A streptococcus to selectively detect the strep A antigen.
|
|
Ultra-sensitive, Convenient, Economical. Convenient. Economical. QuStick eliminates waste with room temperature storage and a two-year shelf life from date of manufacture. You get results in five minutes and, QuStick is not only priced less, it outperforms the leading brand. QuStick Strep A Each kit includes:
STANBIO LABORATORY
1261 North Main Street | | Boerne | TX |
Stanbio Laboratory is committed to manufacture and market a complete line of high quality clinical diagnostic products with an unmatched level of service and support.

|
|
 |
Mono Rapid Test
STANBIO LABORATORY
Test kit provides a fast, convenient, sensitive and economical approach to the detection of Infectious Mononucleosis in whole blood, serum or plasma. Available in a 20 test configuration, the RELY™ Mono Rapid Test kit includes individually pouched cassettes, sample buffer, disposable sample droppers, disposable heparinized capillary tubes with dispensing bulb and positive and negative controls. An easy-to-use procedure card is also included.
|
|
Simple and Easy-to-Use
Sensitivity:
STANBIO LABORATORY
1261 North Main Street | | Boerne | TX |
Stanbio Laboratory is committed to manufacture and market a complete line of high quality clinical diagnostic products with an unmatched level of service and support.

|
|
 |
RELY H.pylori Rapid Test
STANBIO LABORATORY
easy-to-use, two-step whole blood procedure is CLIA waived and requires minimal training. Multiple sample types (whole blood, serum, or plasma) provide efficient testing flexibility. All testing materials are included in the kit to minimize staff hands-on time and convenient room temperature storage and extended dating help manage inventory costs. Available in a 20 test configuration. Test kit includes individually pouched cassettes, sample buffer, disposable heparinized capillary tubes with d
|
|
Simple and Easy-to-Use
Sample:
Sensitivity:
Storage:
STANBIO LABORATORY
1261 North Main Street | | Boerne | TX |
Stanbio Laboratory is committed to manufacture and market a complete line of high quality clinical diagnostic products with an unmatched level of service and support.

|
|
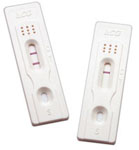 |
True® 20
STANBIO LABORATORY
True® 20 & True® 20 Plus Pregnancy Test - Everything you need is included in one foil pouch (including dropper). The device features a clear, easy-to-read background that ensures reliable results each and every time, providing the highest quality at an afordable price. You will truly notice the diference.
|
|
• Economy version of the QuPID® pregnancy testing device • Sensitive – Detects 20 mIU/mL hCG or greater in both urine and serum specimens • Fast – Positive results may be seen in as little as 10 seconds • Accuracy: >99%, Specificity: >99% • Improved Readability – Quick clearing background ensures easy-to-read results even with low-level specimens • Built-in Positive and Negative Procedural Controls – Ensure proper reagent function and correct testing procedure • Convenient to Use – Specimen dropper packaged with each test device • Room Temperature Storage • CLIA Status: Waived (Urine specimens) • Long Shelf-life
STANBIO LABORATORY
1261 North Main Street | | Boerne | TX |
Stanbio Laboratory is committed to manufacture and market a complete line of high quality clinical diagnostic products with an unmatched level of service and support.

|
|